Ecogenetics
Ecogenetics: The Fusion of Genetics and the Environment
In my book Experimental Man, I had my complete genome sequenced plus extensive testing of levels of environmental toxins (trace amounts) in my body. I investigated what was known about the interaction of genetics with the environment, and concentrated on DNA markers correlated with genetic sensitivity to environmental toxins like mercury. Check out the Experimental Man website for this:
Experimental Man: Environment
Articles about ecogenetics:
National Geographic
The Pollution Within
A Chemical Autobiography
October, 2006
Discover Magazine
How to Tell If You’re Poisoning Yourself with Fish
March, 2009
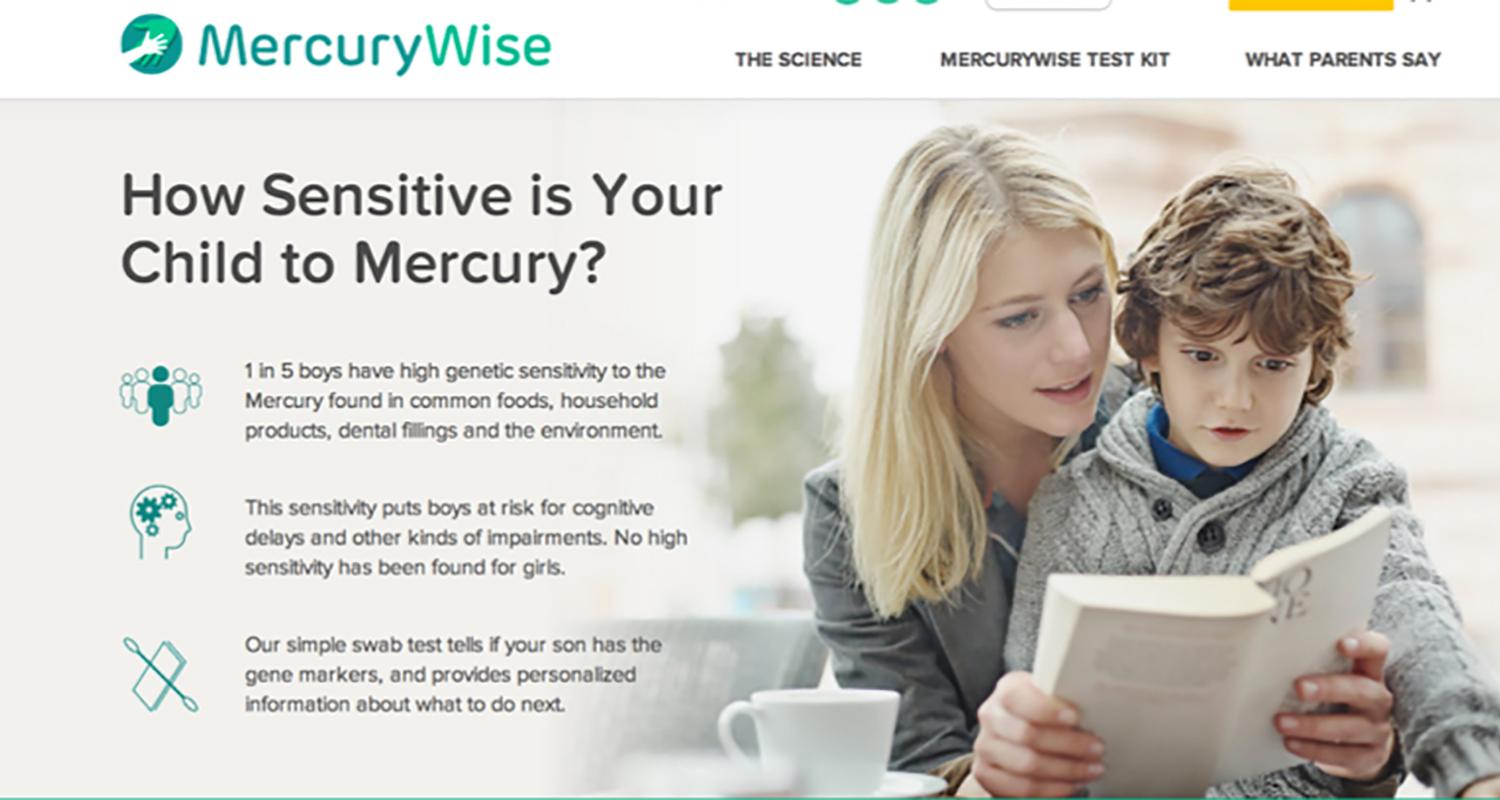
In 2012, I cofounded Ecoeos, Inc., a company that is using deep science to investigate genetic variants that convey an enhanced genetic sensitivity to environmental toxins. The first product being developed is a test for children to characterize a suite of genetic markers that identify genetic sensitivity to low levels of exposure to mercury. Check out the website we have developed:
www.mercurywise.com
(Login: ecoeco Password: eco 14)
Note that this is a prototype site that is not set up to take actual orders.
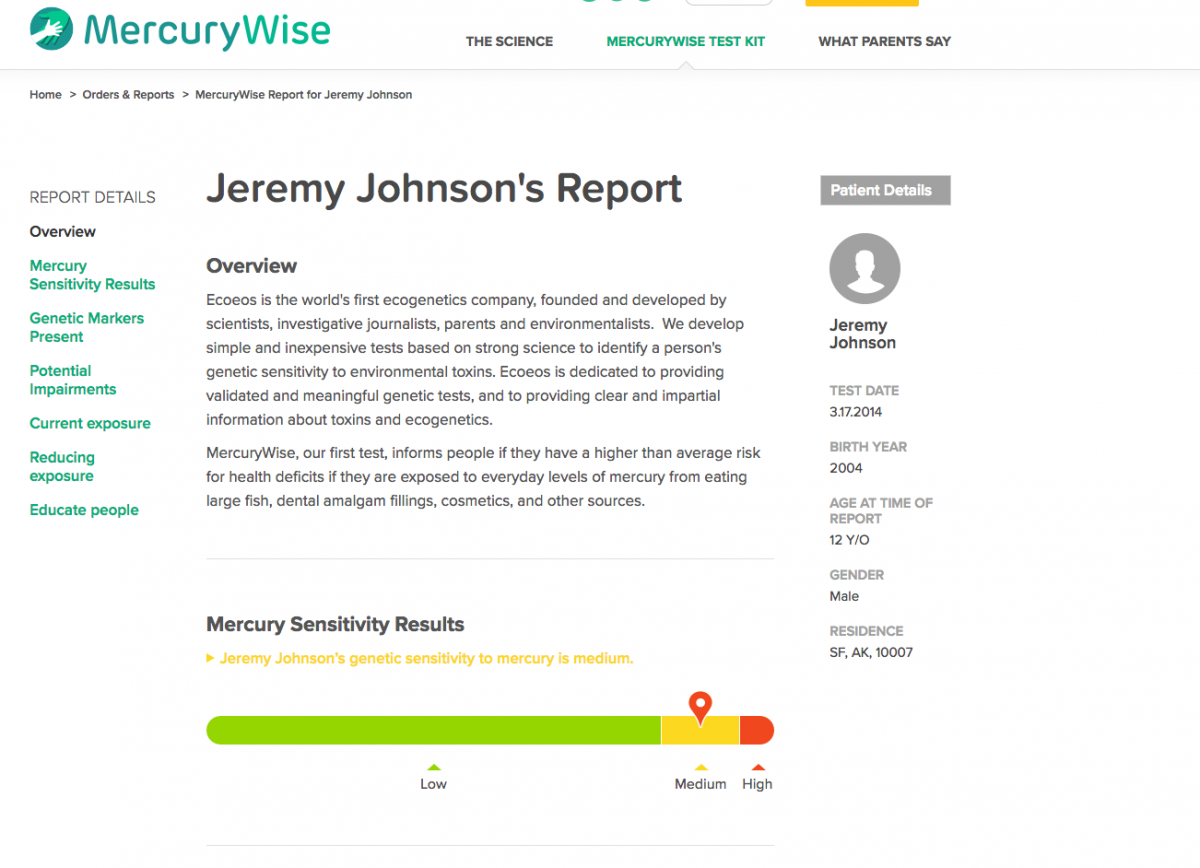
Sample of MercuryWise report and action plan
(Login: ecoeco Password: eco14)
Study that is the basis of the MercuryWise test:
GENETIC POTENTIATION OF RISK FOR NEUROBEHAVIORAL DEFICITS FROM CHILDHOOD EXPOSURE TO MERCURY: ANALYSIS OF LONGITUDINAL DATA FROM THE CASA PIA DENTAL AMALGAM STUDY
Pradeep Babu Sadasivan Pillai, MD, Consulting Research Scientist, Ecoeos,Inc.
James Woods, Ph.D., Research Professor Emeritus, Environmental and Occupational Health Sciences, University of Washington (Toxicologist)
W. James Gauderman, Ph.D., Professor of Preventive Medicine, Director, Division of Biostatistics, University of Southern California, Keck School of Medicine
Evangelos Hytopoulos, Consulting Statistician, Ecoeos, Inc. Gregory Stock, Ph.D., Chief Scientific Officer and Co-Founder, Ecoeos,Inc.
Abstract
Ecoeos researchers and collaborators have found clear evidence for substantially heightened vulnerability to mercury exposure (mercury sensitivity) among children with genetic variants at key loci in five genes. The findings were derived from follow-on research on a 7-year randomized trial (the Casa Pia Study ) on 508 school children in Lisbon, Portugal. In the original study, half of the children (all between the ages of 8 and 17}, were provided with dental care that included amalgam fillings, which are 50% mercury, and half were provided with composite fillings, which contain no mercury. The study tracked the neural development of the two cohorts using a battery of standard neurobehavioral tests conducted annually. Urinary mercury levels were also measured annually. In this original study genetic influences were not investigated. Researchers reported that no statistically significant differences were found between the test scores in the two groups (Rouen, et al, 2006). The follow-on study, launched after the original results were published, looked for possible genetic potentiation of mercury toxicity in 332 of the original subjects – those available to be genotyped Z and examined associations between their test performances and the status of candidate variants at 22 genetic loci. This study found strong, statistically significant deleterious effects associated with specific variants in five genes: CPOX, COMT, MTIM, MT2A, and TDO2. Substantial neurobehavioral deficits (diminutions in excess of 1 standard deviation in mean performance) across multiple neurobehavioral domains including attention, long-term memory, visual processing and fine motor control were found in children with mercury sensitivity who had modest levels of creatinine-adjusted urinary mercury (<5 ug/g). Gender differences were found, boys being far more strongly affected than girls. Approximately one in five boys had multiple genetic-risk markers and was highly mercury sensitive. Boys with high mercury sensitivity and elevated urinary mercury levels, even though these levels were well below what is considered safe by the World Health Organization (WHO threshold = 50 ug/g), showed deficits in multiple performance measures including on average a developmental deficit at age 17 of three to five years in fine motor control and attention, meaning that these children were performing on average at the level of 12 to 14-year olds.
The Casa Pia Study was funded by the Dental and Craniofacial Research Institute at the National Institutes of Health. The original study (1998Z2005) was conducted by a team led by researchers from the Department of Dental Health Services at the University of Washington in conjunction with the University of Lisbon Dental School. The later genetic study (2010Z2013) was initiated by James Woods, PhD, of the Department of Environmental and Occupation Sciences at the University of Washington; the analysis was done by Woods in collaboration with Ecoeos, Inc.
